Introduction
Progressive chronic degeneration of the PR, RPE, BM and choriocapillaris primarily affecting central vision. This is progression risk rises with age.
- Vision loss is usually due to neovascularisation or geographic atrophy
- Worldwide, prevalence for 45yo+ is 8.7%
- Australian prevalence for 50 yo+ is 26.26%
Pathophysiology:
- A combination of factors from both the environment, the lifestyle, normal ageing changes and pathological changes.
- Genetic background, oxidative stress, lipid peroxidation, chronic inflammation, neovascularisation and fibrosis are key features.
8 Changes to Bruch's Membrane with age
1. Progressive thickening occurs
2. Lipid accumulates to restrict nutrient/waste transport
3. Basement membrane thickness means less elasticity or hydraulic permeability
4. Collagen cross-linking means decreased permeability, elasticity, flexibility
5. AGE accumulation decreases protein function
6. Proteoglycan size increases to decrease anti-inflammatory response
7. Elastic Layer calcification causes low elasticity, making the membrane brittle
8. Increased RPE breaking and defects causing choroidal neovascularisation (CNV)
Other effects of ageing:
- Choroidal ischaemia: progressive decrease in choroidal thickness and choriocapillaris density decreases choroidal blood flow. Could lead to hypoxia and up-regulation of angiogenesis.
- Oxidative stress: High metabolic activity by RPE increases ROS. Other factors include smoking

Dead Giveaways
Earliest Sign of AMD:
BLamD --> Basal laminar deposits are ECM material buildup between the RPE and the BM, and is protein and collagen rich
BLinD --> Basal linear deposits between RPE and Bruch's ICL, and is lipid rich
This sign cannot evaluated clinically, only by histology

B shows the BLamD and C(D) shows BLinD
Drusen (Hallmark of AMD):
Deposits between the RPE basal lamina and the Bruch's ICL.
Contains lipid, proteins, cholesterol and carbohydrates
More about drusen is covered in myopic maculopathy
As the first clinical sign, its size is determined by the major inferior venule crossing the disc, which represents approximately 125 microns.

Baseline A small druplet is <63 microns
A medium drusen is between 63 and 125 microns
A large drusesn is >125 microns
Drusen can be reticular (located in the RPE) or cuticular drusen (looks like sawtooth)

Compared to standard drusen (C) underneath RPE, reticular pseudodrusen (D) appears to be in front 
Sabretooth like appearance of the cuticular drusen
Drusenoid Regression: Typically, drusen will grow before regression and undergoing atrophy
Typically, higher drusen loads are associated with higher progression risk
Regression occurs in 20-50% of AMD over 2 years
More likely in eyes with greater baseline drusen and volume
Overtime, these coalesce and becomes larger, with a strong association with late AMD (82%)
All cases of GA/MNV are preceded by drusen regression.
Pigmentary Abnormalities:
Describes the presence of any non-drusenoid hyper/hypo-pigmentation at the macula.
This indicates a risk of progression to late AMD
Hyperpigmentation is due RPE clumping, or increased pigmentation in RPE
Hypopigmentation is due to RPE atrophy and thinning, or loss of pigmentation in RPE
Inflammation (Hallmark of AMD):
Oxidative stress can induce proinflammatoy responses for chronic local inflammation
The presence of chronic local inflammation plays a role in both neo-vascular and non-neovascular AMD
Inflammation is mediated by the complement pathway
Mutations for example in complement H can increase inflammatory response
Excessive cell damage occurs and debris accumulates
Angiogenesis and CNV:
Formation of new BV
VEGF is a key regulator. With the calcification and thickening of Bruch's membrane, combined with a thin choriocapillaris and reduced blood flow, this can lead to retinal hypoxia which releases VEGF
This causes a breakdown of the outer BRB, with the abnormal blood vessels disrupting RPE/BM integrity, creating haemorrhages and fluid leakage.

Classic CNV: Well-defined, early leakage 
Occult CNV: Ill-defined, later and less leakage
Beckman Classification of AMD:
No apparent age changes = No drusen or pigmentary abnormalities
Normal age changes = Small druplets only, but no pigmentary abnormalities
Early AMD = medium drusen with no pigmentary abnormalities
Often asymptomatic with no vision loss
Intermediate AMD = Large drusen and/or ANY AMD pigmentary abnormalities
Reduced CS and dark adaptation, possible visual distortions
iRORA (incomplete RPE and outer retinal atrophy)

Features similar to cRORA but smaller and only at intervals. Typically does not present in geographic atrophy, but is associated with a high risk of impending geographic atrophy
Late AMD = Neovascular AMD and/or ANY geographic atrophy + Requires at least 2 conversion risk factors
Geographic Atrophy: Atrophy of outer retina, PR, RPE and choriocapillaris >250 microns, typically beginning in perifoveal macula. Presents with insidious and gradual vision loss.
cRORA (complete RPE and outer retinal atrophy)

Region of choroidal hypertransmission of 250 microns or more, with RPE attenuation or disruption. Overlying PR degeneration FAF can be very helpful in tracking progression

Patterns can predict
Neovascular: Whilst non-neovascular cases account for 80% of cases, vision loss is slowly progressive. Neovascular cases on the other hand accounts for 20% of cases, but 90% of severe vision loss. Vision loss can be sudden, causing scotoma or metamorphopsia

From Nature, Bae K et al. 2019. Shows the OCT-A of neovascular AMD. Comes with subretinal hyper-reflective material, fibrosis and scarring, PEDs, haemorrhages.
diagnostic features
General Risk Factors:
Old age increases risk. 3x increase for 75 y/o compared to 65
Smoking
Family History
Cardiovascular risk factors (modifiable)
Hypertension
Hyperlipidaemia
Obesity
Nutrition and exercise (low dietary intake of vitamins A, C, and E, zinc, lutein, omega-3 fatty acid.
Genetic Risks:
Potential genetic influence suspected, and strong evidence.
Focuses on genes involved in modulation of the complement system and lipid metabolism
Genetic susceptibility combined with environmental factors contribute to disease onset and progression
Conversion Risk:
Single eye involvment + large drusen = 1 risk factor
Single eye involvement + pigmentary changes = 1 risk factor
Fellow eye involvement + large drusen/pigmentary changes = 1 risk factor
Both eye involvement with medium drusen = 1 risk factor
Late AMD = 2 risk factors
MNV:
Neovascularisation involving the macular
Type 1:
Located between Bruch's membrane and RPE, with ingrown vessels from the choriocapillaris extending into and under the RPE
Appears same as an occult lesion on fluorescein angiography
On OCT, presents as a PED wit heterogenous reflectivity, representing fibrovascular content
May have overlying haemorrhages.
Type 2:
Proliferation of new vessels that extend above the RPE into the subretinal space
Shows a hyper-reflective lesion (well defined) with intense leakage
Appears similar to the classic lesion on angiography
Type 3:
Downgrowth of vessels from the retinal circulation towards outer retina
Also known as retinal angiomatous proliferator or RAP lesion
OCT typically shows extension of hyper-reflectivity from the middle retina to the RPE as well as intraretinal fluid or cystic spaces
Prognostic Biomarkers for AMD:
Used to predict the risk or outcome of a disease in the population without therapy
Hyper-reflective Foci
41% of AMD eyes
Dot-shaped intraretinal lesions at the apex of drusen
Typically corresponds with focal pigmentary abnormalities
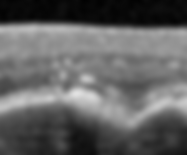
Reticular Pseudodrusen
9-58% of AMD eyes, and appear yellow-white
2-6x higher risk of progression to advanced AMD
Nascent geographic atrophy (iRORA)
7% of AMD eyes
Subsidence of OPl and INL and a hypo-reflective wedge, typically located within the central 1500 microns of macula
Sub-RPE hyper-reflective columns
Unknown prevalence, but appears as a pillar of increased transmission, preceding both GA and CNV

Drusen with subretinal fluid
Present in 11% of eyes. May represent subclinical CNV or mechanical strain

Drusen substructures
Present in 24% of eyes
Non-homogenous internal reflectivity variations within drusen
May be associated with GA